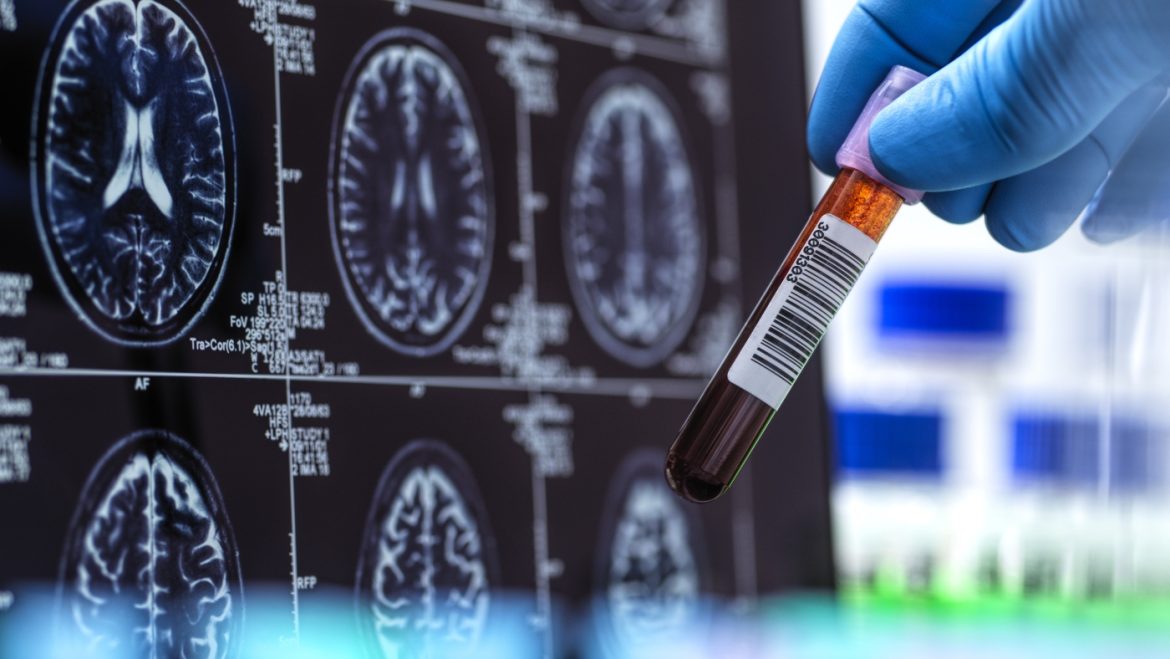
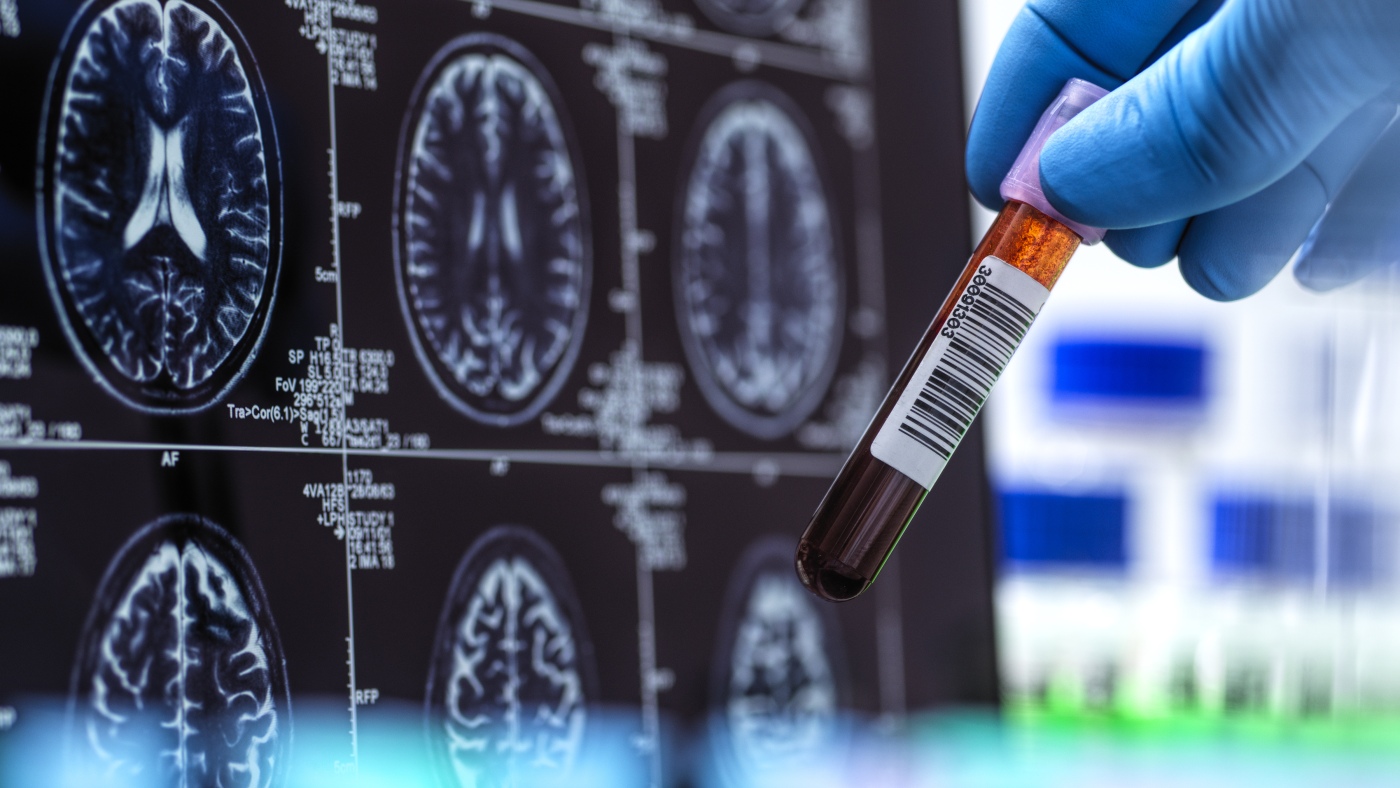
The recent FDA clearance of the first blood test for diagnosing Alzheimer’s disease marks a transformative moment in neurological healthcare. This development promises to make Alzheimer’s diagnoses faster, more accessible, and potentially more accurate, reshaping the current diagnostic landscape and patient experience.
The Diagnostic Challenge of Alzheimer’s Disease
Alzheimer’s disease, a progressive neurological condition characterized by cognitive decline and memory impairment, affects over 7 million older Americans and countless others globally. Traditionally, diagnosis has relied on costly, invasive, or limited-access procedures such as positron emission tomography (PET) scans and cerebrospinal fluid (CSF) analysis via spinal taps. These methods, while effective, are often burdensome for patients and healthcare systems, contributing to delayed diagnosis and treatment initiation.
Introducing the Lumipulse G pTau217/β-Amyloid 1-42 Plasma Ratio Test
The FDA’s authorization highlights the Lumipulse G pTau217/β-Amyloid 1-42 Plasma Ratio test, developed by Fujirebio Diagnostics. This blood-based biomarker assay detects pathological hallmarks of Alzheimer’s, notably amyloid plaques and phosphorylated tau protein (pTau217), both critical indicators of disease presence and progression. Designed for adults aged 55 years and older exhibiting symptoms suggestive of Alzheimer’s, the test facilitates early detection by measuring the plasma ratio of these proteins.
How the Blood Test Advances Diagnosis
– Increased Accessibility and Convenience: Unlike PET scans or lumbar punctures, blood sample collection is far less invasive, less expensive, and can be conducted in a primary care or outpatient setting. This democratizes access to diagnostic tools, making early detection available to a broader population.
– Comparable Accuracy: Research reported by the FDA and multiple clinical trials suggest the Lumipulse test achieves about 92% accuracy compared to PET scans or CSF tests in detecting amyloid plaques. Importantly, in certain analyses, accuracy exceeds 97%, signifying its reliability in clinical use.
– Positive Impact on Clinical Decision-Making: Early and accurate diagnosis enables clinicians to initiate treatments earlier, tailor care strategies, and counsel patients and families effectively. It also allows for better clinical trial enrollment, accelerating Alzheimer’s research.
Implications for Patients, Physicians, and the Healthcare System
– Primary Care Physicians Empowered: Maria Carrillo, Chief Science Officer of the Alzheimer’s Association, emphasizes that this blood test gives primary care providers a faster “read” on Alzheimer’s status, potentially reducing delays in specialist referrals and invasive testing.
– Reduced Healthcare Costs: The affordability of blood testing may help alleviate the economic burden associated with neuroimaging and hospital-based diagnostics.
– Broader Research and Therapeutic Opportunities: Standardizing an accessible diagnostic tool encourages routine Alzheimer’s screening in symptomatic individuals, potentially leading to advancements in disease-modifying therapies as patients are identified earlier in their disease course.
Limitations and Considerations
While promising, the FDA clarifies that plasma testing is not intended to serve as a sole screening or definitive diagnostic tool. It complements but does not replace the comprehensive clinical evaluation, including physical examination, cognitive testing, and other relevant assessments. Moreover, the test is specifically cleared for adults over 55 exhibiting symptoms, not as a broad screening test in asymptomatic populations.
Future Outlook: A Paradigm Shift in Alzheimer’s Care
The FDA clearance of this blood test heralds a major step forward toward practical, early Alzheimer’s detection. It underscores a shift from reliance on complex imaging and invasive sampling to a more patient-friendly and scalable approach. Anticipated availability in clinical practice from June onward in the U.S. will likely prompt rapid adoption.
As this test integrates into standard care, it is expected to facilitate earlier therapeutic interventions, improve patient outcomes, and empower caregivers with timely information. Furthermore, it may catalyze innovations in biomarker research and personalized medicine approaches tailored to neurodegeneration.
—
Conclusion: Revolutionizing Alzheimer’s Diagnosis with Blood-Based Biomarkers
The introduction of the FDA-cleared Lumipulse blood test for Alzheimer’s disease signifies a potent leap toward faster, more accurate, and accessible diagnosis. By transforming a historically complex and invasive process into a simple blood draw, it offers hope for millions of older adults and their families confronting cognitive decline. This breakthrough elevates the standard of neurological diagnostics, opening pathways to improved care, research, and ultimately, a future where Alzheimer’s disease can be detected and treated more effectively from its earliest stages.