Tailor-Made Gene-Editing Treatments Break New Ground in Rare Genetic Disorders
The landscape of medicine is witnessing an extraordinary transformation with the advent of personalized gene-editing therapies. Recently, a medical milestone was reached as doctors administered the first-ever bespoke CRISPR gene-editing treatment to a baby born with an exceptionally rare, life-threatening genetic disorder. This landmark achievement not only embodies hope for the affected infant but also paves the way for bespoke genetic treatments for thousands of rare diseases that have, until now, had little or no effective therapies.
Understanding the Promise of Gene-Editing for Rare Disorders
Rare genetic diseases often stem from specific mutations disrupting normal gene function. Traditional treatments generally manage symptoms but rarely target the root genetic cause. Gene therapy, especially gene-editing technologies like CRISPR, is revolutionizing this by allowing precise modifications to the patient’s DNA. This form of intervention can potentially correct the defective gene itself rather than merely mitigating downstream effects.
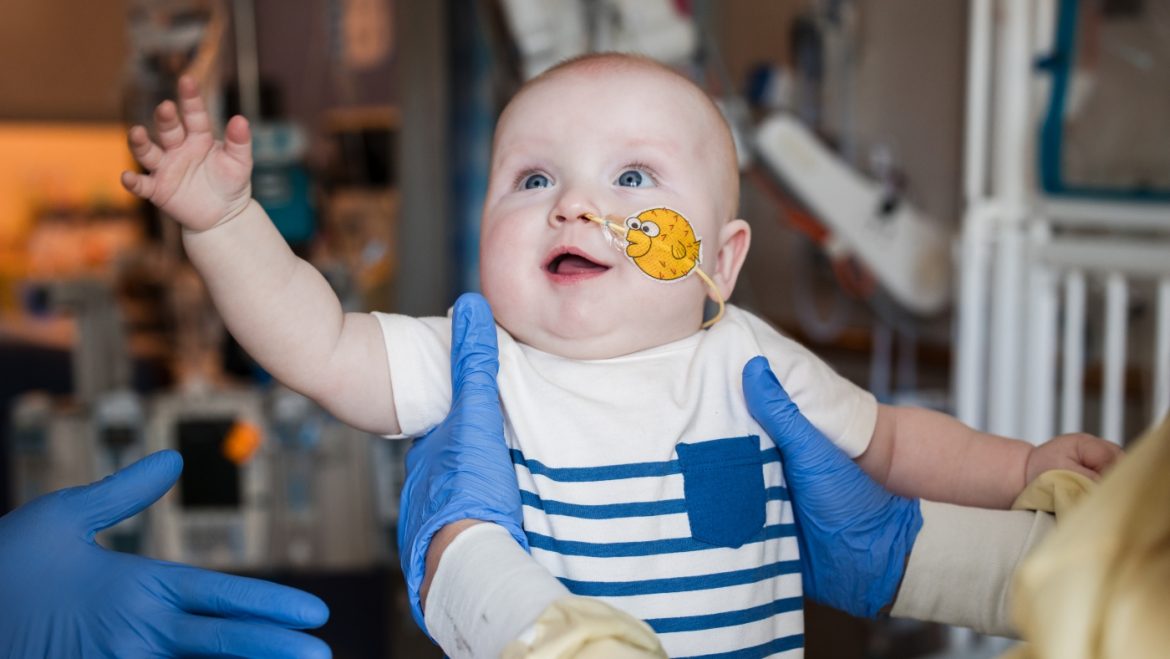
The case of the infant, KJ Muldoon, exemplifies this scientific leap. Born with a severe and ultrarare metabolic genetic disorder, KJ’s condition threatened serious neurological damage and a bleak prognosis. However, within six months of diagnosis, a custom CRISPR-based therapy was developed specifically targeting his unique mutation. This therapy involved billions of gene-editing particles designed to home in on the defective gene and repair it at its source.
Tailoring Treatments: The New Frontier
What makes this case unprecedented is the tailor-made nature of the treatment. Rather than a one-size-fits-all approach, the therapy was created after sequencing and understanding KJ’s specific genetic mutation. This bespoke design adjusted the gene editing tool to address exactly the mutation causing his disease, maximizing the therapeutic efficacy and minimizing off-target effects.
The process included identifying the mutation, engineering a CRISPR system to cut and repair the gene precisely, producing the treatment, and administering it in timely infusions. This entire pipeline, which historically could take years, was compressed into six months by leveraging recent biotechnological advances and collaborative clinical infrastructure.
Clinical Outcomes and Broader Implications
Following treatment, KJ showed remarkable improvement, thriving without the severe symptoms that were predicted before therapy. The success signals a new paradigm—not just treating symptoms but potentially curing diseases inherited from birth through immediate genetic correction.
Furthermore, such tailored gene-editing therapy illustrates a scalable blueprint for addressing other rare genetic conditions. Many of these disorders, like metachromatic leukodystrophy (MLD), AADC deficiency, or carbamoyl-phosphate synthetase 1 deficiency, affect newborns and toddlers and currently lack effective treatments. This breakthrough points to a future where rapid sequencing and bespoke CRISPR design become standard practice.
Challenges and Ethical Considerations
Despite this progress, challenges remain. The complexity and cost of creating a unique therapeutic for each patient are formidable. For instance, some approved gene therapies such as Libmeldy® for MLD carry multi-million-pound price tags. There are also safety considerations, as gene therapies can involve viral vectors with inherent risks or unintended gene edits.
Ethical questions around gene editing—especially when applied prenatally—also demand ongoing discourse. Careful oversight is required to balance innovation with patient safety, equitable access, and societal values.
Advances Beyond Postnatal Treatment: Prenatal and Early Intervention
Complementing these postnatal treatments, scientists have also begun exploring treating genetic diseases before birth. By injecting therapeutic molecules, like antisense oligonucleotides (ASOs), into the amniotic fluid or applying gene therapies in the womb, doctors aim to prevent irreversible damage in fetuses diagnosed with severe genetic disorders.
Such early intervention approaches have shown promise in motor neuron diseases and other fatal conditions, significantly improving survival rates and quality of life. The integration of prenatal diagnostics with gene therapy highlights the growing synergy of genetics, molecular biology, and neonatal medicine.
A Vision for the Future: Personalized Medicine on Demand
The rapid development of personalized gene-editing therapies heralds an era where infants born with rare diseases might no longer face inevitable decline. Advances in genomic sequencing technology, gene-editing methods, and manufacturing processes are converging to shorten the time from diagnosis to treatment dramatically.
Moreover, national healthcare systems are beginning to integrate these therapies (e.g., NHS England’s provision for gene therapies for fatal genetic disorders), expanding access beyond clinical trial settings. As production becomes more efficient and regulatory frameworks mature, more families may benefit worldwide.
Conclusion: From Medical Firsts to Routine Lifesaving Therapies
The treatment of KJ Muldoon with a personalized CRISPR therapy is a beacon of hope that the “incurable” genetic disorders of the past will not dictate outcomes for future generations. It demonstrates what is possible when cutting-edge science meets urgent medical need—and sets a precedent that personalized gene editing can be both feasible and life-saving.
As the field progresses, the focus will likely shift toward streamlining bespoke treatment development, ensuring safety, expanding prenatal applications, and addressing the economic challenges. This first success story is not merely a rare event but the opening chapter in a revolution that could redefine rare disease treatment forever. For countless children born with debilitating genetic disorders, personalized gene-editing therapies now hold the promise of a thriving future previously thought unimaginable.